+7 (929) 727 53 60 Traumatology / Orthopedics / Neurosurgery
DOCUMENTS AND LICENSES
Information letter of the rector of the SamGMU Kotelnikov G.P.pdf Certificate of Conformity dentistry.pdf
Certificate of Conformity traumatology.pdf Declaration of Conformity Traumatology.pdf
Informed voluntary consent for patients.pdf A letter from RosZdzdravNadzor on individual blocks. PDF
1. Registration documents and licenses, technical conditions. Quality of materials
The use of bio-implants "Lyoplast" ® approved by the Ministry of Health.
TU-9398-001-01963143-2004 Registration Certificate FS 01032004 / 1567-05 dated April 29, 2005.
The collection of donor material is carried out in accordance with the Law of the Russian Federation “On Transplantation of Organs and Tissues” No. 4181-1 of November 22, 1992.
License of the Russian Federation М99-01 -0021 04 dated February 9, 2006
Requirements for use, transport and storage:
- Biotechnologies are transported by all types of covered vehicles in accordance with the rules for the carriage of goods, operating on the appropriate type of transport.
- Type of dispatch: by postal parcel and all modes of transport (by road, air and rail) according to GOST 20435 or GOST 18477.
- Bioimplants transportation conditions - according to storage conditions 5 (ОЖ4) GOST 15150.
- Bioplant in the packaging of the manufacturer should be stored in storage conditions 1 GOST 15150. at a temperature of + 1 ° C to + 30ºС.
- Each bioimplant must be packed in an individual double-package film-film, film-paper, manufactured by Wiburiy oy wipak medical, Finland, Baxter, or in glass vials from 5 to 100 ml.
- Packaging film-film, film paper should be hermetically welded with the labeling of the package film-film indicator gamma sterilization (Color indicators are selected according to GOST R 50325-92).
- The material packed in glass bottles should be hermetically sealed with a rubber stopper and an aluminum cap (GOST R ISO 10993, OST 64-2-82-85, GOST R 51314-99).
- Individual packs with bioimplants must be packed in quantities from 1 to 5 in a box made of cardboard GOST 9142, GOST 9481, GOST 13511, GOST 13512 ,. GOST 13513, GOST 13516, GOST 13841.
- After opening the package, the bioimplant should be used and unused material should be disposed of.
- Shipping containers with bioimplants are pasted over with polyethylene tape with a sticky layer according to GOST 20477 or glue tape on paper basis GOST 18251 or GOST 10459 paper.
Contents of delivery:
- bioimplants -1 pc.
- consumer packaging -1 pc.
- label - 1 pc.
- application instruction -1 pc. on group packing.
2. Preliminary analyzes and tests of material and potential donors
Bioimplants obtained using the “Lyoplast” technology consist only of components of the human body and do not contain chemicals introduced from outside.
All donors undergo autopsy and serological blood tests for syphilis and hepatitis B and C viruses, AIDS.
The production technology of bioimplants “LYOPLAST” allows the recipient to be fully protected from the transmission of any disease to him, minimizes the risk of infection to personnel and makes the process environmentally safe and economical.
Serological tests are carried out to all donors before tissue sampling.
Serological testing for antibodies to pale spirochete:
- Express analysis;
- Complement binding reaction.
Serological tests for markers of viral infections:
- HBsAg;
- AntiHCV;
- Antibodies to HIV.
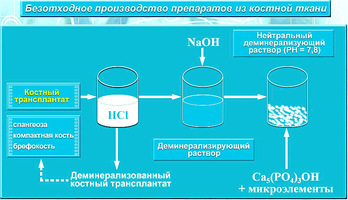
3. The original technological complex multi-stage cleaning material Lyoplast
In the manufacture of “Bioimplants for bone allogenic mechanically and ultrasound, the processed lyophilized dental sterile“ LYOPLAST ”® uses the original cadaver tissue fabrication algorithm.
In the production process, mainly physical factors are used, and the use of chemical reagents is minimized.
At the first stage, the process of obtaining bioimplants includes a special ultrasonic treatment of tissues for removing elements of bone marrow and fat, carrying out primary sterilization of the material, and viral inactivation.




After preprocessing, the tissues are lyophilized, and then the hermetically packed material is sterilized by radiation.
Processing of bioimplants using physical factors:
- Vacuum
- Ultrasound 26 - 40 kHz
- Lyophilization
- Gamma sterilization (fast electron sterilization).
4. Technical requirements, requirements for safety and quality of materials, sterilization
Bioimplants must comply with the requirements of technical conditions and manufactured according to the technological regulations № 028, approved in the prescribed manner.
Bioimplants should be made on the basis of tissue of allogeneic nature.
Preparation and processing of bioimplants is carried out according to the Appendix to the order of the Minister of Health of the USSR dated June 14, 1972. No. 482.
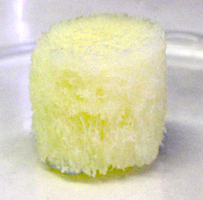
Bioimplants should be manufactured in accordance with the shape, size and volume, according to the specifications:
- The surface of bioimplants should be without cracks, without break-offs and shells.
- The color of bone bioimplants should be white, gray or ivory.
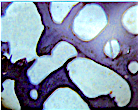
- The relative content of lipids in cancellous bone tissue is (1.2 ± 1)%.
- The moisture content in bioimplants should be no more than 5%.
- Hydrogen indicator (pH) of bioimplants should be in the range of 4.0-8.0 pH.
- The dispersion of the powder should be in the range of 103-101.
- Bioimplants must be sterile.
The sterilization method is radiation. Dose of sterilization (20,0 ± 5,0) kGrey.
Individual packaging of bioimplants should be airtight.
Bioimplants during transportation must be stable in transport packaging to the effects of climatic factors for storage conditions 5 (ОЖ4) GOST 15150.
During transportation, bioimplants must be resistant to mechanical influences in transport packaging (vibration loads: frequency range 10-55 Hz, displacement amplitude 0.35 mm; shock loads: peak impact acceleration 10g, impact acceleration duration 16 ms).
The average shelf life of bioimplants should be 3 years.
5.Manufacturer's warranty. Unconditional and stipulated warranty. Responsibility

The manufacturer guarantees the compliance of bio-implants "Lyoplast" with all the requirements of these specifications, subject to the conditions of transportation, storage, operation, established by these specifications.
Warranty period of validity 3 years from the moment of sterilization.
Lyoplast bioimplant and component warranty program
1. Warranties for Lyoplast bioimplants and components.
1.1 Bioimplants Warranty. LYOSELL provides a guarantee for the whole shelf life of bioimplants from the date of sterilization.
1.2. The warranty on the components of bio-implants "Lyoplast". LYOSELL provides a guarantee for the whole shelf life of bioimplants from the date of sterilization.
1.3. Exemption from warranty. The warranties covered by this document apply only to reproducible and certifiable defects and do not apply to products: bioimplants, their components or other products, or any services provided by anyone other than LYOSELL, or LYOSELL offices listed on the official site lyoplast.com.
2. Providing guarantees and warranty support.
2.1. The date of commencement of guarantees. For users of Lioplast bioimplants, the guarantee period starts from the first day after delivery of the goods.
For users purchasing products in the online store or through personal contact, a warranty certificate is issued for a specific patient at each purchase.
2.2. Notification. The user of bio-implants "Lyoplast" is obliged to properly notify by telephone or e-mail the company "LYOSELL", as well as the representative office "LYOSELL", indicated on the official website lyoplast.com directly about any problem associated with bio-implants and components, as well as provide information sufficient to establish the true causes of the problem.
2.3. Support by a single phone (964) 342-16-12.
Clinical telephone support service is available free of charge from 9:00 to 18:00 local time for all users of Lioplast bioimplants who are working on the territory of the Russian Federation acting at the time of treatment.
After receiving the request, the support service diagnoses the problem and offers a solution by phone.
If this is not enough to make a rational decision that falls under the current Supply Contract and the Clinical Support Service cannot solve the problem remotely, then a date will be set for the LYOSELL representative to meet with the doctor and patient to find out the reasons and fill in the Questionnaire on the bioimplant and component exchange. .
3. Responsibilities of the user of Lyoplast bioimplants
3.1. The right of the user of Lioplast bioimplants on the Warranty in accordance with this document takes place only if the user of the Lyoplast bioimplants fulfills the following obligations:
- ensure that operations are carried out in accordance with the recommended implantation protocol and with the use of the necessary tools and equipment,
- keep tools and equipment in proper condition, ensure high-quality and thorough cleaning and chemical treatment, proper sterilization in accordance with official recommendations of manufacturers;
- not to make any changes and not to apply with products not related to bioimplants "Lyoplast" without the prior written consent of LYOSELL,
- contain “Lioplast” bioimplants, their components in purity and integrity, use them properly and carefully in accordance with the instructions for use developed by LYOSELL, and do not allow to work with bioimplants and components of unauthorized or not trained personnel ;
- contain the outer surfaces of the packaging of bioimplants and components in a pure form and under normal conditions,
- do not attempt to adapt or modify the Lyoplast bioimplants and do not ask, authorize or authorize anyone other than LYOSELL and other LYOSELL offices listed on the official website of lyoplast.com to carry out such a device for working with Lyoplast bioimplants
- use bioimplants, their components only for performing implantation operations, surgical procedures and manipulations with Lyoplast products,
- do not use any accessories, application or additional equipment, except those that were provided or approved in writing by LYOSELL.
3.2. Changes. LYOSELL can, at its discretion, provide the user with certain structural and technological “Changes” of Lyoplast bioimplants and their components, and the user of Lyoplast bioimplants hereby undertakes to properly use the Changes and in accordance with the instructions and instructions provided.
LYOSELL will update and replenish the product line of bio-implants Lioplast and regularly inform the user during the validity period of the current Delivery Agreement.
3.3. Register on the web site lyoplast.com.
The user of bio-implants "Lyoplast" is obliged to register on the website of the Company "Lyosell" (lyoplast.com) specifying the registration data in accordance with the electronic form, which is an indispensable condition for providing the Guarantee to the user of bio-implants "Lyoplast".
In the absence of such registration, LYOSELL has the right to refuse to perform this Lyoplast Guarantee Program on bioimplants and their components in whole or in part.
3.4. The end user of bioimplants and their components.
The user of bio-implants "Lyoplast" guarantees that he is an end-user of bio-implants "Lyoplast" and their components, acquired or received for use in accordance with the current Supply Contract, and that the products (bio-implants, their components, and other products and services of LYOSELL as well as other representative offices of LYOSELL, indicated on the official website of lyoplast.com, were not acquired for the purpose of resale or distribution, and also undertakes not to allocate, transfer or transfer in any way about bioimplants, their components and other products and services of LYOSELL and other LYOSELL representative offices listed on the official website of lyoplast.com, or the Guarantee without the written consent of LYOSELL and other LYOSELL representative offices listed on the official website site lyoplast.com.
The rights of the user of bio-implants "Lyoplast" under the current Delivery Agreement cannot be transferred to third parties without the consent of LYOSELL and other LYOSELL offices indicated on the official website lyoplast.com.
4. Disclaimer of Warranties.
Termination of the obligations of the LYOSELL Company as well as other LYOSELL offices indicated on the official website lyoplast.com
4.1. LYOSELL does not bear warranty obligations and does not produce a free exchange of bioimplants and their components in the following cases:
- if the product intended for personal use has been used for other purposes inconsistent with its intended purpose ;
- violation of the rules and conditions of operation of the installation of the product, as set out in the Quality Certificate "Lyoplast" and other documentation, transferred to the patient complete with the product;
- if the product has traces of unqualified correction attempts ;
- if the complication is caused by the treatment performed in another clinic;
- if the complication is caused by the action of irresistible forces, accidents, deliberate or careless actions of the consumer or third parties ;
- if mechanical damages occurring after the transfer of the product to the end user are detected; damage caused by exposure to moisture, high or low temperatures, corrosion, oxidation, ingestion of foreign objects, substances, liquids, other objects;
- if the defect occurred due to normal wear and tear during use of the product and its packaging has been lost.
- In this case, natural wear refers to the consequences of using the product, which caused a deterioration in their functional state and appearance due to the long-term active use of this product; LYOSELL will not be liable for possible damage, directly or indirectly caused by the product to people, pets, or property, if this occurs as a result of non-compliance with the rules and conditions of operation, installation of the product; deliberate or careless actions of the buyer (end user) or third parties.
To obtain a warranty exchange of bioimplants and their components, you can contact the support phone at +7 (964) 342-16-12 or another specified in the Warranty Card.
Scientific basis and promising developments related to plastic materials Lyoplast
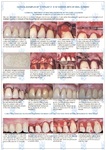
During the existence of the Samara Bank of Fabrics, all types of bone-substituting materials using the Lioplast technology have been clinically tested in many cities of Russia and the European Economic Community.
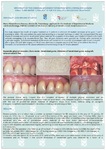
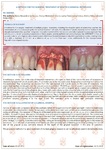
Doctors use materials in dentistry: when removing teeth for the preservation of the holes, in periodontal surgery, for implantation of teeth, bone plastics, for the treatment of periimplantitis, periodontitis; to increase the volume of the jaw bone in width and height, in orthodontics.
In maxillofacial surgery and traumatology, Lyoplast bioimplants are used for prosthetics of entire bones, replacement of the mandible, for extensive injuries and resections.
Every year there are new patents on the types of materials Lioplast and methods of their use.
Currently, new forms of production are being developed (ring-shaped blocks, bone cones, curved dura mater, and many others) to maximize the individualization of bioimplants and simplify the operation of bone grafting and dental implantation.
For standard products it is planned to change the packaging to a more convenient one (single syringes).
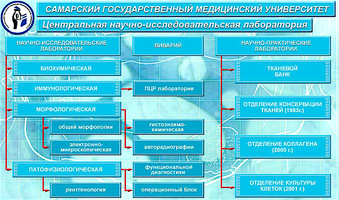
Tight interaction with the staff of the vivarium, biotechnological and histo-morphological laboratories help in scientific research, as well as confirmation of laboratory and primary clinical results in animal experiments and histological studies.
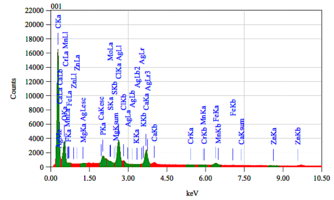
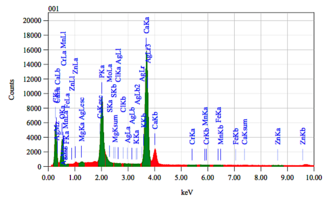
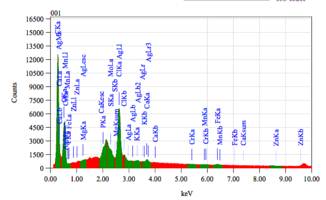
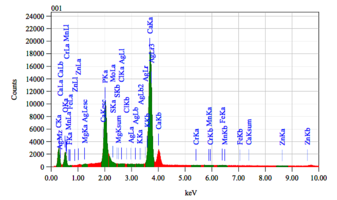
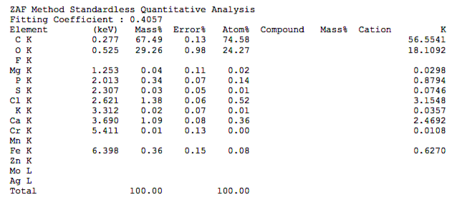
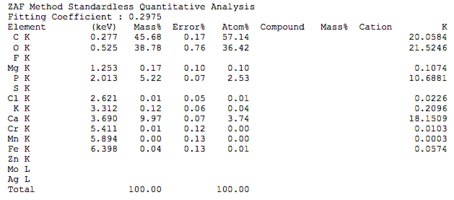
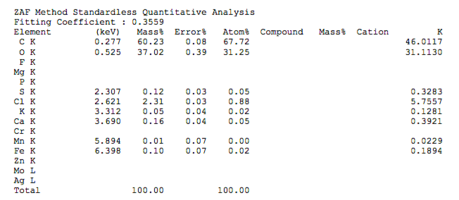
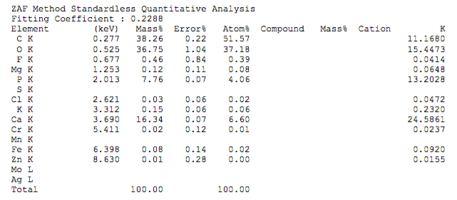
Chemical composition of demineralized cortical powder.pdf
The chemical composition of demineralized spongy powder.pdf
The chemical composition of mineralized powdered powder.pdf
The chemical composition of mineralized cortical powder.pdf
Priority reference surgical technique for individual bone blocks.pdf
Priority reference pharmacotherapeutic support of bone grafting.pdf